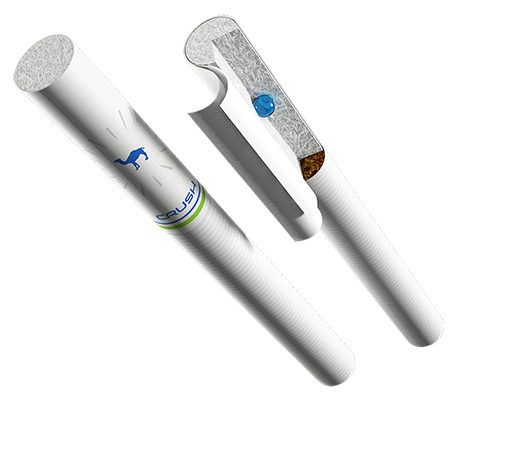
Modern producers in pharmaceutical industry continuously develop technologies for production of the multicomponent medicines having certain properties master the latest technologies which priority question is increase in efficiency of medicinal substances and safety. Encapsulation of medicinal substances in a cover is extremely perspective and popular method of regulation of their properties. It is necessary to notice, already rich history of technologies of encapsulation, they are widely used not only only in chemical pharmaceutical industry, but also in agriculture, in the food and chemical industries and other otrasly branches. The review of technologies of encapsulation for production of various dosage forms is provided in this article: firm and soft, gaseous and liquid. The concept of encapsulation (Latin of capsula — a box) means the conclusion of fractional particles of a solid body and their units (granules), or liquids (thaw) in thin, but quite strong cover (or a matrix) with the various predetermined properties, such as ability solubility or not solubility in different environments, the melting temperature, permeability, etc. Pharmaceutical industry differentiates the processes allowing to make softgel capsules of 10.1 in size — 10.4 cm: encapsulation of menthol cigarette softgel capsules of the big sizes (0,5 — 1,5 cm) and microencapsulation. The purposes of encapsulation of medicinal substances are: preservation of unstable medicines in vitamins, antibiotics, enzymes, vaccines, serums, etc. from harmful effects of factors of the external environment; masking of unpleasant taste and smell of medicinal substances; an obespechivaniye of release of medicinal substances in the caused department of a GIT (kishechnorastvorimy microcapsules); the obespechivaniye of the extrapolated medicine influence, i.e. the slowed-down release of small doses of an active component supports its certain level in an organism and the most effective therapeutic influence for an appreciable length of time; combination of incompatible purely medicinal substances in one medicine (with use of partitive dividing coverings); transfer to pseudo firm condition of gases and liquids (loose weight from the microcapsules filled with gaseous or liquid medicinal substances with a firm cover); swallowing simplification; simplification of further processing, in particular in the high-speed packing line. The encapsulated substance (the main component of microcapsules) can be in any aggregate state: liquid, firm, gaseous. Modern methods of microencapsulation give the chance to apply both liofilny, and liofobny substances. Microcapsules may contain the exicipient representing the environment of dispergating of substance during microencapsulation or necessary for further functioning of active agents. The quantity in microcapsules of the encapsulated substance is, as a rule, 50 — 95% of the lump of softgel capsules. This size can vary according to conditions of receiving and technology, the required ratio of the encapsulated substance and material of covers and other conditions of process: viscosity of the environment, temperature, availability of surfactants, extent of dispergating, etc. The term "microcapsules" ("nanocapsules") can designate a number of different structures. Use of the molecules detaining active agents inside, or combinations of difficult molecules which form nanocapsules (nanosphere) subsequently is possible. Nanoencapsulation takes place if the size of molecules does not surpass several micrometers. If the size of a molecule does not exceed one millimeter, then it is about microencapsulation. Substances of various classes can act as the cover material (encapsulating matrixes): Lipids and wax: bee, karnubsky, kandelilsky wax, wax emulsions, the natural and modified fats, glitserol distearate. Carbohydrates: sucrose, starches, glucose, maltodextrins, chitosan, alginates, ethyl-cellulose, atsetattsellyuloza, etc. Proteins: wheaten and soy proteins, zein, gluten, gelatin, etc. Both proteins, and their modifications are used. Degradiruyemy polymers: polybutadiene, polyvinyl acetate, polypropylene, polystyrene, etc. Depending on mission and properties of the encapsulated substance and an order of its release, and it is equal also from the chosen technology of microencapsulation, selection of materials of a cover or the encapsulating matrix is carried out. Mechanical destruction of covers of microcapsules releases their contents: melting, friction, pressure, ultrasonic influence, breaking from within vapors or gaseous substances which are emitted from change of external conditions, diffusion of contents of the microcapsule when swelling its walls in surrounding liquid, interaction with Wednesday (at dissolution in it) cover substances. It is conditionally possible to divide the existing microencapsulation methods into three main categories: a) the physical methods of microencapsulation based on mechanical ways of formation of a cover. In this category of methods — extrusion with use of centrifuges or the forming " devices a pipe in a pipe", drawing a covering in a fluidized layer, vacuum dusting (condensation of vapors). b) the chemical methods based on the chemical transformations leading to production of film-forming material — sewing together of polymers for formation of a new phase, polymerization and polycondensation. As high-molecular (polymers and oligomer), also and low-molecular substances can be exposed to chemical transformations. c) physical and chemical methods — sedimentation of film-forming polymer from the water environment by method of addition of a component for reduction of its solubility, a koatservation, generation of a new phase at changes of temperatures, hardening of fusion in liquid environments, evaporation of easily flying solvent, extraction replacement, physical adsorption, drying with dispersion. Several major factors need to be considered at the choice of a method of microencapsulation. From them the most important is the mission of a product, the defining conditions of use of the encapsulated substance and manifestation of its property. The choice of film-forming material and the predetermined environment for microencapsulation depend on this factor. The method of diffusion causes the slowed-down release of substance and demands use of the film-forming material which is bulking up, but not dissolved in the environment of use of microcapsules. In need of fast release of substance, it is possible to choose the soluble, melting or fragile film-forming material. One more factor — solubility and stability in the conditions of microencapsulation of the encapsulated substance. Instability Many substances, such as easily flying liquids, some vitamins, enzymes, are unstable even at insignificant temperature increase. It limits admissibility of use of the methods providing heating. As alternative, it is possible to apply methods at the heart of which - division of liquid phases (education from solutions of a new phase). Properties of substance will define at the same time the choice of a disperse phase and the dispersive environment. The cost of process is of great importance, in this regard those methods which are carried out in the continuous mode are most preferable and include smaller number of stages. Also the efficiency of microencapsulation, the estimated size of microcapsules and content of the encapsulated substance in them are important. At the heart of the above described classification of methods of microencapsulation (rather conditional) the nature of processes which result at microencapsulation is put. In practical activities the complex of various methods is often applied. Further we will consider microencapsulation methods, the most widespread in chemical pharmaceutical industry.
Capsulation in the theory.
training materials.